In Jacob Hanna’s lab at the Weizmann Institute, for the first time in the world, scientists succeeded in growing a mammal embryo outside the womb. The implications of this unprecedented achievement could be enormous: From growing synthetic embryos for organ implants to creating an infant whose biological parents are two men. No less
Israeli scientists have grown 250-cell embryos into mouse fetuses with fully formed organs using artificial wombs, in a development they say could pave the way for gestating humans outside the womb.
“We have grown hundreds of mice in this way, in a method that has taken seven years to develop, and I’m still captivated every time I see it,” stem cell biologist Prof. Jacob Hanna of the Weizmann Institute of Science told The Times of Israel.
“This could be relevant to other mammals including humans, though we acknowledge that there are ethical issues related to growing humans outside the body,” he said.
Hanna said that his research will advance understanding of organ formation in mammals — and could facilitate medical advances — as it allows unprecedented views of the process unfolding, unconstrained by the need to image the inside of the uterus.
His breakthrough was revealed on Wednesday in the peer-reviewed journal Nature.
While scientists have been trying for decades to grow mammals outside the body, success has been limited to either very early-stage embryos that are grown in a lab for a short period or fetuses that were removed from the womb once their organs are formed and then grown in a lab.
When the Children’s Hospital of Philadelphia created an artificial womb in 2017 that successfully grew fetal lambs for over four weeks, the lambs already had their organs at the start of the experiment.

But Hanna started with embryos consisting just of stem cells, and watched “with amazement” as the organs of the mice — animals that have a speedy gestation of just 19 days — grew in front of his eyes.

“We took mouse embryos from the mother at day five of development, when they are just of 250 cells, and had them in the incubator from day five until day 11, by which point they had grown all their organs.
“By day 11 they make their own blood and have a beating heart, a fully developed brain. Anybody would look at them and say, ‘this is clearly a mouse fetus with all the characteristics of a mouse.’ It’s gone from being a ball of cells to being an advanced fetus.”
The fetuses were healthy but died at 11 days, as this is currently the longest they can develop in the artificial womb and they can’t be transplanted back into a mice uterus. But Hanna hopes to develop his technology to take mice to full term.
His method involves placing the embryos in a special liquid to nourish embryo cells in a laboratory dish and getting them to float on the liquid. With this step, they succeeded in duplicating the first stage of embryonic development, in which the embryo grows tenfold.
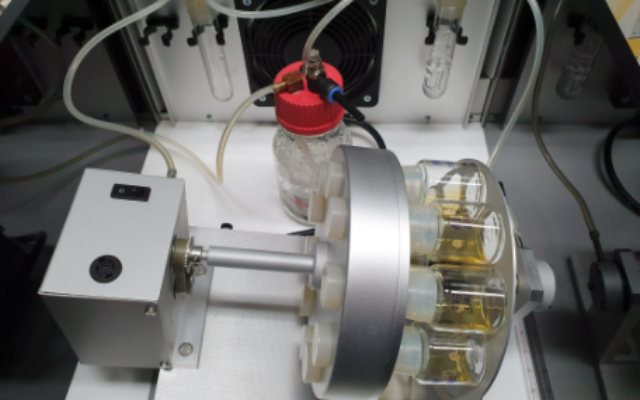
“The key to our success is that we have developed this special incubator system in which each embryo is in a bottle with liquid, and the bottle is spinning to ensure it doesn’t attach to the side. The incubator creates all the right conditions for its development.
“What made this possible is the seven-year journey which has seen us develop the liquid, which really gives the embryo all the nutrients, hormones and sugars they need, and the incubator, a custom-made electronic device which controls gas concentration, pressure and temperature.”
 ionigeria.com Nigerian Newspaper
ionigeria.com Nigerian Newspaper

